In recent years, lithium-ion batteries have become the essential power source for smartphones, power tools, electric vehicles, and energy storage systems. While many people recognize their “large capacity and long lifespan,” few understand the science behind them. This article provides a simple and clear explanation of how lithium-ion batteries work and why they dominate modern energy technology.
I. Basic Structure of Lithium-ion Batteries
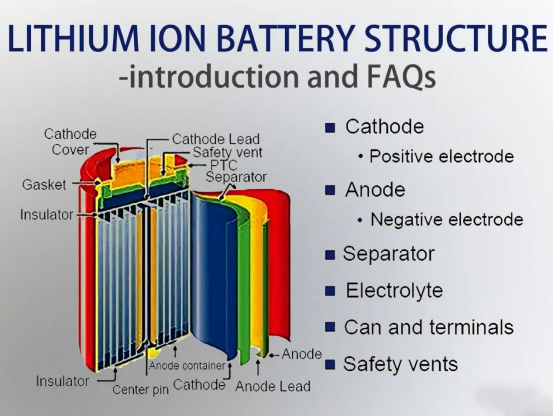
A lithium-ion battery is composed of several critical components that together form a closed yet reversible electrochemical system:
1. Positive Electrode (Cathode)
Common materials include:
NCM (Nickel-Cobalt-Manganese)
LFP (Lithium Iron Phosphate)
2. Negative Electrode (Anode)
Typical materials:
Graphite
Silicon-carbon composites
3. Separator
A microporous membrane that prevents short circuits while allowing ions to pass through.
4. Electrolyte
A conductive liquid that enables lithium-ion transport between the electrodes.
5. Battery Casing & BMS (Battery Management System)
Provides structural protection, temperature monitoring, charging control, and safety.
II. How Lithium-ion Batteries Store and Release Energy
The core operating principle of lithium-ion batteries is the back-and-forth movement of lithium ions between the positive and negative electrodes.
• During Charging
Lithium ions move from the positive electrode to the negative electrode.
They are stored within the anode material.
• During Discharging
Lithium ions travel back to the positive electrode.
This movement generates the electrical current that powers devices.
The reversibility of this process allows lithium-ion batteries to maintain performance across hundreds to thousands of charge cycles.
III. Why Lithium-ion Batteries Outperform Lead-acid Batteries
Lithium-ion technology has rapidly replaced traditional lead-acid batteries due to several key advantages:
1. Higher Energy Density
Stores significantly more energy within the same volume.
2. Lighter Weight
Ideal for portable electronics, tools, and electric vehicles.
3. Longer Lifespan
Typically delivers 500–3000+ charge cycles, depending on chemistry and usage.
4. Lower Self-Discharge Rate
Retains power even after long periods of inactivity.
IV. Future Trends in Lithium-ion Battery Technology
The development of lithium-ion batteries continues to accelerate, with major innovations on the horizon:
1. Solid-State Batteries
Higher energy density, improved safety, and longer cycle life.
2. Cobalt-Free Batteries
More sustainable and safer chemical systems.
3. Smart Energy Storage Systems
Integration with smart grids to enhance renewable energy usage and stability.
Conclusion
Lithium-ion batteries have transformed modern technology and will remain a dominant force in consumer electronics, transportation, and energy storage for the next decade and beyond. With continuous improvements in safety, energy density, and sustainability, they will play an even greater role in shaping the future of global energy.